
Platonic Relationships
This 36” diameter spherical painting, known as a Termesphere®, by Dick Termes is a study of the five regular polyhedra also known as the Platonic Solids. Geometers have studied the Platonic Solids for thousands of years because of their fascinating interconnectivity and logical beauty.
Platonic Solids are polyhedron whose faces are congruent regular polygons, and where the same numbers of faces meet at every vertex. The best known example is a cube whose faces are six congruent squares.
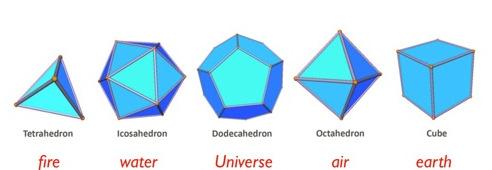
The five polyhedra that are part of this are the tetrahedron, octahedron, cube, icosahedrons and the dodecahedron. How do they relate to each other? If you create a icosahedrons ( twenty adjacent equilateral triangles) and find the center of each triangle and connect these points to each other you create the dodecahedron (twelve adjacent pentagons). If you take one vertex of the dodecahedron and draw an line between that vertex, skip a vertex and go to the next vertex and continue that to the next pentagons you will get a square. If this is continued around the whole dodecahedron you will produce a hexahedron or cube (six adjacent squares). From the cube you can produce the last two polyhedra. The octahedron ( eight adjacent triangles) is found by adding center points to the square faces of the cube and connecting these points together. The tetrahedron (four adjacent triangles) is found by taking the squares of the cube and drawing diagonals to each of the squares. This creates a tetrahedron. This shows how they all relate to each other.
I made the triangles, pentagons and the squares of these polyhedron into circular tubes of different colors and different thicknesses when I created PLATONIC RELATIONSHIPS.
Plato believed the four regular polyhedron were the structure of the atoms, water, air, earth and fire. It was not true but we have found out they are the shapes of packed atoms and molecules like crystals. Sodium chloride is cubical and calcium fluoride is octahedral and pyrite is dodecahedra. So much of the early thinking for chemistry came from these solids.
Kepler 1571 – 1630 spent a large part of his life studying how these solids made up the spacing of our solar system. This was a great study even if it turned out to be untrue.